
Many antigens may be analyzed in parallel under the very same conditions without needing to biochemically purify or renature the proteins, An extra TAG, e.g. undecapeptide epitope, fused, in a specific embodiment, into the C-terminus of every antigen allows the detection and quantification of any full size protein antigen bound to the ELISA plate using one monoclonal antibody.
If no sign is detected, check the following:
a) confirm that proper antibody clones were utilized;
b) assess the action of this enzyme/substrate procedure: e.g., coat 1 µg/ml of biotinylated detection antibody in many molds in binding buffer to get a few. Wash a few times proceed with the ELISA routine from Measure 13 after blocking. In the event the system is busy, then a sign ought to be viewed;
c) confirm the action of normal or attempt a sample of regular. 6,7 Due to this enzyme-mediated amplification of the detection antibody indicate, the sandwich ELISA can quantify physiologically relevant (ie, > 5-10 pg/ml) concentrations of particular cytokine and chemokine proteins, that are found in combined cytokine milieus, e.g., from stimulated lymphocyte culture supernatants. By making serial dilutions of a normal protein solution of focus, A curve is integrated into a sandwich ELISA assay.
Seal with tape and incubate the plate. Seal with tape and incubate the plate.
The catch ELISA of the present invention may easily be adapted to several other protein antigens from different viruses, bacteria, certain tumour antigens or some additional protein to which antibody diagnostics are related. The creation of a catch protein to get, e.g. GST, by crosslinking, e.g. glutathione, to casein has a lot of benefits: Low backdrop response with certain cells in biological fluids of several species, particularly individuals; lack of at least reduced binding actions with different proteins, higher solubility and native conformation of the purified protein antigen in physiological buffer systems and higher stability during longterm storage. Generic catch Elisa for detecting antibodies in 13, using fusion proteins.
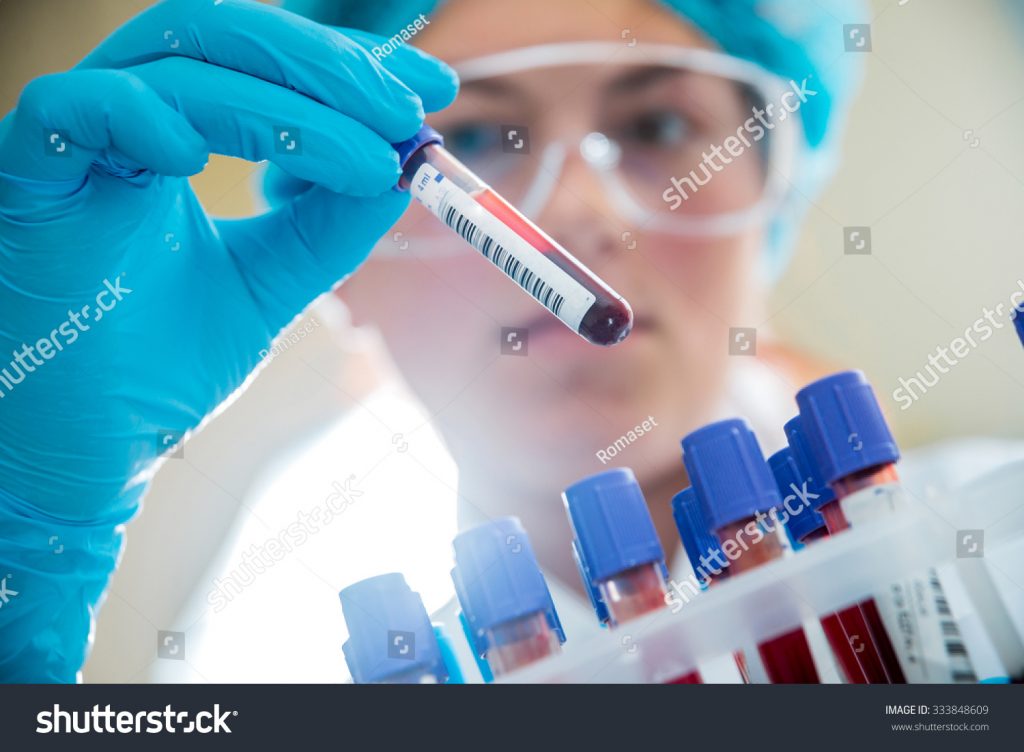
A sandwich ELISA steps antigen involving two layers of Compounds (detection and capture antibody). Coat wells of top binding polystyrene microtiter plates (catalogue # S2506710, Immulon Thermoflecton Corporation, Milford, MA, USA) together with Ag catch agent (protein) or restrain blocking buffer (BSA) -50µl of 2µg /ml protein antigen /Coating Buffer solution also, tap and shake to coat molds.
The process not only enables effective and specific detection of antibodies in biological trials however, moreover, easy and effective immobilization and one-step elimination of overexpressed recombinant antigens from crude lysates on ELISA plates coated with, e.g., glutathione casein.
The process enables the effective and specific detection of antibodies in samples however, moreover, elimination and effective and easy immobilization of recombinant antigens that are overexpressed even from lysates on ELISA plates coated with the . Simultaneous assays (where the two sample and antibody are added simultaneously to the sound substrate-bound antibody) or inverse sandwich assays (in which the labeled antibody and sample to be analyzed are combined, incubated and inserted into the unlabelled surface bound antibody) may be utilized for the discovery of hepcidin in biological trials. The analyte can also be referred to as the land since it’s going to specifically bind or ligate into a detection reagent, thus ELISA drops under the larger group of ligand binding assays 2 The ligand-specific binding reagent is”trapped,” i.e., usually dried and coated on the translucent bottom and at times additionally side wall of a nicely 6 (the static”solid phase” /”solid substrate” here instead of solid microparticle/beads which may be washed off ), which is generally built as a multiple-well plate called the”ELISA plate” Conventionally the specificity of antigen – antibody type response is utilized since it’s simple to raise an antibody specifically.
When chromogenic substrate is added to the assay to come up with colour, samples with higher antigen concentration generate greater signal compared to those with reduced antigen concentration, making a signal directly proportional to the quantity of antigen from the sample. Minus the very first layer of”catch” antibody, any proteins in the sample (such as serum proteins) may competitively adsorb into the plate surface, reducing the number of antigen trapped. Immunoassays for detecting antibody or antigen have been developed.
This measure employs the BSA in way to bind to some staying free binding sites in each microwell,’obstructing’ the detection antibodies, antigen or other samples elements from binding non-specifically into the plate. The indirect or sandwich ELISA provides a remedy for this issue, using a”catch” antibody specific for the test antigen to pull it from this serum’s molecular blend.
Two ). Steps integrated to permit for the elimination of endogenous protein and antibodies included using a secondary antibody before incubation. In Short, detection of specific antibodies is eased by the coating of microtiter plates using particular antigen followed by the incubation of diluted evaluation bleed or undiluted supernatant samples.
The usage of antigen-specific discovery and capture monoclonal antibody increases the sensitivity and specificity of the assay (in comparison to indirect ELISA)
Afterward, the focus of the particular allergens sample was estimated by multiplying the concentration found in the chart by the dilution factor.
We anticipated that the variety of the target antigen and antibody concentration by utilizing different dilutions. Dilution of a sample extract is essential to get an ELISA which subsequently determines the worth of the array for target and antibody antigen concentrations.
The colour intensity is based upon the concentration of wheat protein within the particular sample and quantified with an iMark microplate reader (Bio-Rad) at 405 nm. The Microplate Manager (MPM) program was used while assessing the optical frequency values of both samples and standards. The ELISA system has been implemented to detect antibodies. Antibody reactivities of sera from patients with cervical carcinoma and wholesome people have been in good agreement with those determined with a previously recognized capture ELISA using biochemically purified and renatured proteins as antigens even though the catch ELISA of the current invention was sensitive without the lack of specificity.
Remove these serum samples and wash the wells using the wash buffer using a wash bottle or a ELISA plate washer (1) time. Bridging ELISA is a unique instance of a sandwich ELISA where a dimeric or oligomeric antigen (most frequently an antibody in a sample) is discovered by means of a discovery and capture antibody. Unpurified Compounds (eg ascites fluid or antiserum) may necessitate increased concentration of this sample protein (attempt 10 μg/mL) to compensate for the reduced concentration of specific antibody.
The overall principle of the assay entails three steps: beginning with catch, or immobilization, of their target analyte on a micro plate, followed by the discovery of the analyte by target-specific detection proteins, and last, receptor response, in which the conjugated receptor exerts its substrate into a colored item. The antigen is”sandwiched” between the capture antibody and another”detection” enzyme-conjugated antibody – in which the two electrons are special for the identical antigen but at various epitopes (3). Selecting the sort of ELISA to do, indirect is dependent upon a range of factors, including the complexity of the Compounds as well as the samples.
Unlike conventional ELISA systems, the CaptSure DIY ELISA kit includes a ready-to-go CaptSure assay plate, pre-coated together with our CaptSure antibody, which is unique for its CaptSure peptide. That the CaptSure DIY ELISA lets you lower the quantity of catch antibody needed, the capability to quantify targets on precisely the plate using antibody pairs, if wanted and enhance your ELISA into some format. CRP spiked in 1: 100 individual blood or serum is supplied and simmer for 15 minutes at room temperature that contributes to the creation of complex, which washed twice with washing buffer and then is captured by the magnet.
But, an antigen is recorded
to the plate (by direct adsorption into the surface or via a pre-coated”catch” antibody, like in a sandwich ELISA), it’s the detection measure (as either indirect or direct detection) that mostly determines the sensitivity of an ELISA.
From the quantitative sandwich ELISA, dilutions of a known benchmark, in this instance recombinate Individual TNFalpha, were inserted to some 96-well plate and also read together with the anonymous samples. For its ELISA assay, the presence of influenza A virus antibodies diluted samples of serum – . The target analyte is an antigen, which discovered by the detection antibody and can be recorded on the plate forming an sandwich.
(APTMS), (3-mercaptopropyl) trimethoxysilane (MPTMS) or 3-glycidoxypropyltriethoxysilane (GOPTS), whereby said binding alternative comprises rather of APTES in a rate of 0.1 to 10%, preferably 0.5 to 5 percent, more preferably 1 percent. The immobilisation method thus comprises the steps of: creation of hydroxyl groups in the substrate by KOH pre-treatment; a single – measure covalent binding of capture reagent (dispersed in 3-aminopropyltriethoxysilane (APTES)) into the protruding element; rather followed by obstructing the non invasive protein binding sites by bovine serum albumin (BSA). In this embodiment, the top element may contain 24 protruding components, whereas the 24 protruding components are incubated in sample, then washed twice in 2 individual sets of 24 wells, then immersed in detection substrate prior to diagnosis, allowing evaluation of 24 samples at a rapid and effective manner utilizing one 96 well microtiter plate. Samples with higher antigen concentration create a signal than those comprising antigen concentration when substrate is added to come up with colour.
Furthermore, the standardized arrangement of this antigen creation and demonstration in the capture ELISA of the present invention can be adapted to identify large chain of antigens in specified geometry on initial binding partner/casein coated solid supports (antigen arrays”) and might enable to ascertain the antibody status of a person sample to a lot of antigens in parallel (antibody profiling”) at one response and manipulation. Ultimately, an individual could also envision many additional in vitro software such as an ELISA of the current invention that may detect antibody amounts from experimental tissue culture media or purification trials or the discovery of antibody degree in biological fluids aside from serum like urine, lymph, and spinal fluid etc.. The catch ELISA of the current invention isn’t just helpful for discovering particular antibodies in samples such as Zinc but can also detect and measure antibodies in biological fluids aside from serum like plasma, urine, saliva, lymph, tears, milk, semen, and spinal fluids, ascites, peritoneal and other effusions and vaginal secretions and to lab fluids like tissue culture supernatans or cell lysates.
A significant drawback of this direct ELISA is that the procedure of antigen immobilization isn’t specific; if serum is used as the origin of test antigen, all proteins in the sample can stick with the microtiter plate well, therefore tiny doses of analyte in serum should compete with other serum proteins when binding to the cell surface. The sample using an unknown quantity of antigen is immobilized onto a solid support (normally a polystyrene microtiter plate) either non-specifically (through adsorption to the surface) or specifically (via catch by a different antibody specific to the identical antigen, in a”sandwich” ELISA).
Poor reproducibility of replicate or standard samples or significant colour in the Negative Control Lines (high non invasive binding worth ) are frequently the outcomes of inadequate washing or may result from contamination of the TMB substrate or splashing of their conjugate. Since the ELISA may be conducted to assess either the existence of antigen or the existence of antibody in a sample, it’s a practical instrument for determining hemoglobin antibody concentrations (like with the HIV test 24 or even West Nile virus). Additionally, it has found applications in the food sector in discovering potential food allergens, such as peanuts, milk, walnuts, walnuts, and eggs 25 and as serological blood test for coeliac disease 26 27 ELISA may also be utilised in toxicology as a quick presumptive display for certain types of medication.
The high degree of signal generated if the substrate is inserted will likely be directly proportional to the quantity of antigen recorded in the plate and jumped by the detection reagents. Capture and detection antibodies which don’t interfere with each other and may bind simultaneously are known as”matched antibody pairs” and are acceptable for creating a sandwich ELISA. When creating a new ELISA maximize the requirements for your antigen or catch antibody and the initial step is to ascertain an plan.
Following plate washings, the trapped radicals function to specifically catch secreted cytokine proteins within samples that were implemented to the plate. Within this ELISA variant, the experimental sample is”sandwiched” between an unconjugated capture antibody and a conjugated detection antibody, each of which are unique to the identical protein but at various epitopes.
Subsequently, 200 μΙ_ of TMB substrate was supplied to every MTP well along with the enzyme-substrate response was allowed to go for 4 minutes before being stopped by 100 μΙ_ of the stop solution. The sandwich immunoassay process contains fewer process steps as the complex is formed in 1 step, once the sample is offered containing biotinylated detection antibody.
The eELISA sandwich immunoassay process, as explained herein, has fewer process steps in comparison with classic ELISA, as the immune complex is formed rather in one measure, whereas the analyte sample is offered to the MTP wells containing biotinylated detection antibody preconjugated into HRP-labeled streptavidin. The sandwich ELISA that was popular and most commonly used is based on the discovery of antibodies. From the ELISA test, the sample antibody is sandwiched between the antigen coated onto also an enzyme-labeled globulin conjugate along with the plate.
The detection antibody may be covale
ntly linked to a molecule or may itself be discovered by means of a secondary antibody that’s connected to a molecule via bioconjugation Between each step, the plate is normally washed with a mild detergent solution to remove any proteins or antibodies which are non-specifically bound. Figure 13. (A) Schematic of the formerly developed 1 -measure antibody immobilization process of sandwich ELISA for CRP (European Patent Application No. WO/2014/056896 – this strategy is employed to immobilise the affinity capture reagent into the compacted component, not the microtitre plate as shown in the structure ), (B) Detection of CRP at PBS buffer (with 0.1% BSA) and CRP- spiked dilute human embryo with standard microplate reader-based readout. Immunometric assays, also referred to as sandwich ELISAs (enzyme-linked immunosorbent assay), utilize two antibodies specific to the antigen to catch or”sandwich” antigen from the nicely for detection.
Gentaur Toutch Microplate Absorbance Reader, using a wavelength Assortment of 400-750 nm, is an economic, high-performance alternative for a wide Assortment of programs, such as immunoassays with colorimetric substrates, including ELISA, along with protein assays like Bradford and Lowry. In serological evaluations, two-fold differences in dimensions of copies of exactly the exact same sample are thought of as acceptable (38). Studies clarify the allergen protein concentration to be analysed by the standardization of dilution factor for jojoba seed test.
[Gentaur_linking template=”rowtwo” type=”products” search=”Elisa” showImage=”false” showDescription=”true” showCatalogNumber=”true” showSize=”true” showPrice=”true” showSupplier=”true” classTable=”blueTable” header=”3″ start=”1″ limit=”1000″]